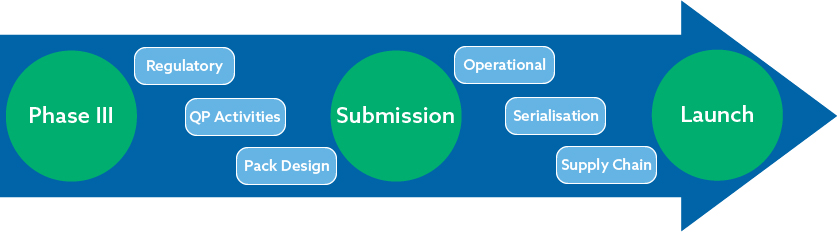
- Learn about the European regulatory framework and how this determines all your launch milestones.
- Understand the importance of the Qualified Person (QP) and QP declarations.
- Discover key EU packaging considerations.
- Gain insights into managing a complex supply chain.
- Learn about the key Serialisation readiness requirements when partnering with a CMO.
- Identify the top operational considerations as you progress to commercialisation.
Not only will our experts share their product launch knowledge and experience but they will be hosting specialist roundtable discussions to address your specific product launch queries.
These roundtable discussions will give you the opportunity to speak with our subject matter experts and ask them more specific questions relevant to your particular project.
Round table session 1
Regulatory & Quality
Operational & Packaging
Round table session 2
Supply Chain & Serialisation
Operational & Packaging
Please visit Workshop sponsor’s website for more detail at:
Event Information
Event Topic:
EU Product Launch — A Workshop
Event Description:
Launching your valuable drug product can be a daunting process, especially if you are not familiar with the key milestone requirements that should be met at various time points in the MA submission process. During these knowledge sharing workshops, our subject matter experts will guide you through the product launch timeline. Taking you from Phase 3 to commercial launch, we will share with you the key requirements and considerations to ensure your commercial launch is successful.
Date/Time:
Date(s) - 09/16/19
9:00 am - 3:30 pm
Event Location:
Other: Hilton San Fran Airport Bayfront
Event Details
Event Type
